Introduction to Cancer Xenograft Models
Cancer xenografts are widely used in preclinical oncology to study human tumor behavior in a living system. By implanting human cancer cells into immunodeficient mice, researchers can observe tumor development, evaluate treatment efficacy, and investigate mechanisms of drug resistance in vivo. This method provides a more accurate representation of how tumors respond to therapies compared to traditional in vitro experiments. Xenografts have become a foundational tool for translating laboratory research into potential clinical applications.
Relevance to Brain Cancer Research
In the field of brain oncology, xenograft models play a critical role in advancing our understanding of tumors such as glioblastoma and neuroblastoma. These cancers are highly aggressive, often resistant to treatment, and difficult to model effectively outside of a living system. Xenografts derived from brain cancer cell lines allow for the evaluation of therapeutic agents under realistic biological conditions, making it possible to test drug delivery, tumor penetration, and long-term treatment outcomes. Both subcutaneous and orthotopic xenograft systems are used to study tumor growth, angiogenesis, and gene expression profiles in real time.
Integration with Transfection-Based Research
The combination of in vivo xenograft models with gene transfection technologies creates a powerful platform for functional studies and drug development. Transfection reagents enable the targeted introduction of genetic material into brain cancer cells, allowing researchers to study the roles of specific genes or therapeutic targets before validating their effects in xenograft models. This progression from in vitro gene modulation to in vivo tumor modeling supports a more complete preclinical workflow, linking molecular discovery to translational outcomes.
A172 Xenograft Model
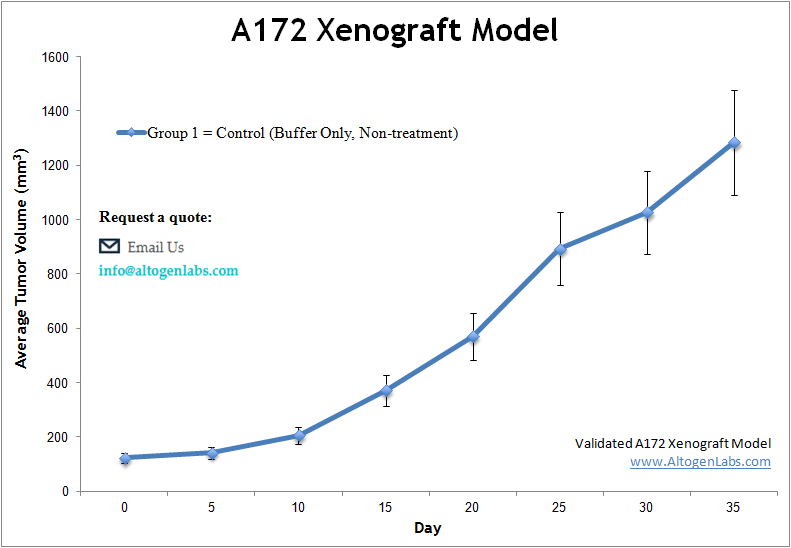
The A172 xenograft model is a human glioblastoma in vivo system used extensively in preclinical cancer research to evaluate drug efficacy, tumor growth inhibition, and targeted therapy mechanisms. Offered as a full-service study platform by Altogen Labs, this model involves subcutaneous implantation of A172 cells into immunodeficient mice, producing reproducible, slow-to-moderate growing tumors that retain key molecular features of high-grade gliomas.
A172 cells are derived from a human glioblastoma multiforme (GBM), one of the most aggressive and treatment-resistant cancers of the central nervous system. These cells express markers such as GFAP and show responsiveness to therapeutic compounds affecting the PI3K/AKT and MAPK pathways—features that make the A172 xenograft system highly suitable for evaluating novel small molecules, kinase inhibitors, and combination therapy regimens targeting glioma-specific vulnerabilities.
Relevance of A172 Xenografts in Brain Tumor Research
While orthotopic brain tumor models are essential for studying tumor–brain microenvironment interactions, subcutaneous xenograft systems using glioblastoma-derived cells like A172 offer a faster and more scalable method for early-stage screening. The A172 model provides a consistent tumor take rate and allows for non-invasive monitoring of tumor volume over time, making it ideal for evaluating treatment responses, establishing dose–response relationships, and comparing drug candidates prior to moving into intracranial studies.
This model has also been used to investigate radiation sensitization, immune pathway regulation (in humanized or co-engrafted systems), and the effects of angiogenesis inhibitors on tumor vasculature. With proper experimental design, it can serve as a foundational platform in the preclinical pipeline for brain cancer therapeutics.
Model Characteristics and Growth Behavior
A172 xenografts are known for forming well-vascularized, moderately aggressive tumors when implanted in athymic mice. The tumors exhibit epithelial morphology and a compact, cellular histology that resembles moderately differentiated gliomas. Compared to faster-growing models like U87, A172 xenografts grow at a more controlled rate, allowing for longer treatment timelines and longitudinal data collection.
In vivo, these tumors often display limited necrosis and low spontaneous ulceration rates, making them suitable for longer studies that involve multiple treatment cycles or survival monitoring. This controlled growth behavior also allows for detailed molecular profiling over time, providing valuable data on treatment-induced gene expression changes and tumor adaptation.
Applications and Customization
The A172 xenograft model is commonly used to support IND-enabling studies, pharmacodynamics analysis, and drug combination optimization. Because A172 tumors tend to grow more slowly than some other glioma models, they are well suited for long-term treatment protocols or extended survival studies. The model can also be adapted for testing nanoparticle formulations, gene therapy vectors, or RNA-based therapeutics.
Altogen Labs works with clients to customize dosing schedules, drug administration routes (including oral, intraperitoneal, or intravenous), and biomarker analysis endpoints based on the study’s scientific goals.
Request an Instant Quote: https://altogenlabs.com/request-quote/
Learn more: A172 Xenograft Model
LN-229 Xenograft Model
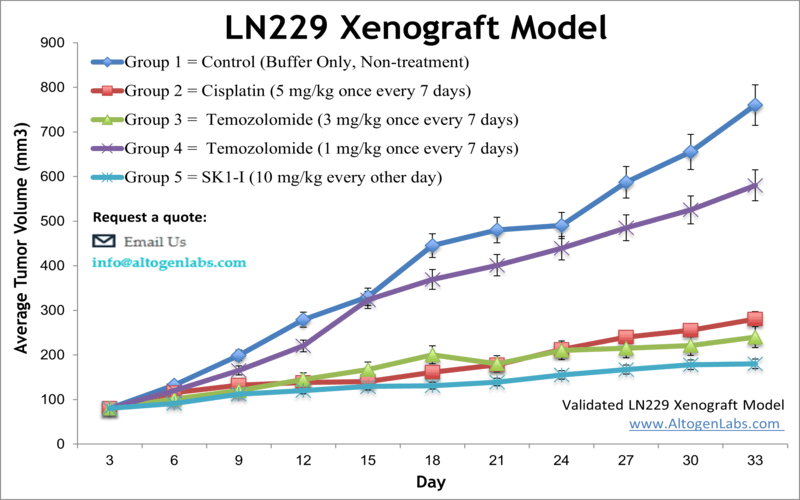
The LN229 xenograft model is an in vivo glioblastoma research system developed using the human LN229 glioma cell line, a well-established tool in neuro-oncology. Offered by Altogen Labs as a fully managed xenograft study service, this model enables preclinical testing of anti-cancer agents targeting key pathways involved in glioblastoma multiforme (GBM). When implanted into immunocompromised mice, LN229 cells form solid, vascularized tumors that recapitulate critical features of invasive brain tumors, making this xenograft model valuable for drug efficacy evaluation, combination therapy development, and mechanistic cancer research.
LN229 cells are derived from a human temporal lobe glioblastoma and possess functional p53 signaling—a feature that distinguishes them from other glioma models like U87 and A172. This makes the LN229 model particularly relevant for testing compounds that modulate p53 activity, DNA damage responses, and apoptosis-related pathways.
Scientific Significance of LN229 in Glioma Research
Unlike many glioma-derived lines that are p53-deficient or highly mutated, LN229 cells retain a partially functional p53 axis and exhibit elevated basal levels of pro-apoptotic signaling. This enables researchers to study interactions between DNA repair mechanisms, chemotherapy-induced apoptosis, and cell cycle regulation. In addition, LN229 xenografts are highly responsive to agents that target the PI3K, MAPK, and EGFR pathways, providing a versatile platform for evaluating a broad range of targeted therapies and immunomodulatory compounds.
Because of their well-documented response to temozolomide and radiation, LN229 xenografts are frequently used to study chemoresistance, glioma recurrence, and radiosensitization strategies. The model supports both single-agent and combination therapy protocols, and can be integrated into longer-term survival studies or biomarker-driven research workflows.
Tumor Characteristics and In Vivo Behavior
LN229 tumors grow at a moderate rate when established subcutaneously in athymic nude or SCID mice. These tumors typically exhibit a dense, cellular morphology with well-formed vasculature, making them suitable for measuring anti-angiogenic activity and drug delivery kinetics. Unlike U87 tumors, which often grow rapidly and with limited infiltration, LN229 xenografts demonstrate more consistent histopathological features and a better correlation with human GBM behavior.
This model also supports extensive molecular profiling over time, including analysis of DNA repair enzyme expression, apoptotic markers such as cleaved PARP and caspase-3, and downstream effectors of stress-response pathways. These characteristics make the LN229 xenograft system ideal for translational research and IND-enabling preclinical validation.
Preclinical Applications and Custom Study Design
The LN229 xenograft model is employed across a wide range of oncology applications, from target validation to pharmacodynamics and resistance pathway studies. It is frequently selected for evaluating experimental treatments involving small molecule inhibitors, biologics, radiation sensitizers, and epigenetic modulators. The model is also amenable to testing nanoparticle delivery systems or RNA-based therapeutics under customized treatment schedules.
Altogen Labs provides comprehensive study design and execution, including cell implantation, tumor volume monitoring, treatment administration, and endpoint data collection. Studies can be tailored to match specific drug regimens, dose escalation designs, and analysis endpoints such as immunohistochemistry, RT-PCR, or protein-level biomarker detection.
Request an Instant Quote: https://altogenlabs.com/request-quote/ln-229-xenograft-model-services/
Learn more: LN-229 Xenograft Model
SF-268 Xenograft Model
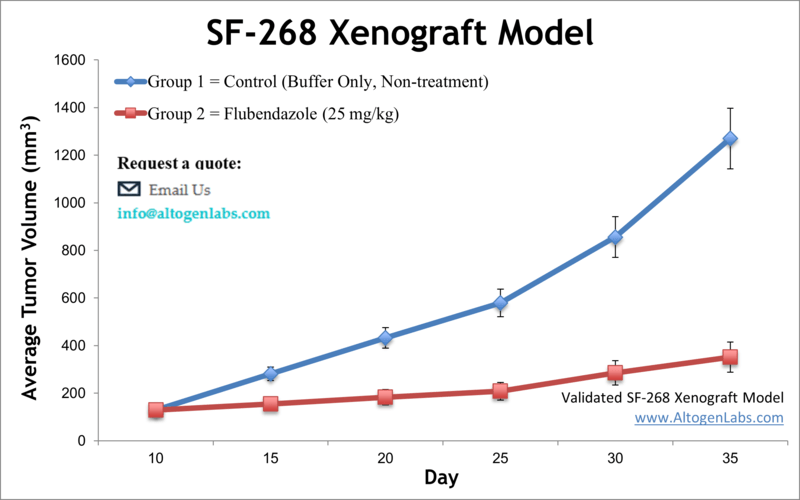
The SF-268 xenograft model is an in vivo glioblastoma multiforme (GBM) system derived from the human SF-268 cell line, used to investigate tumor progression, treatment resistance, and therapeutic efficacy in high-grade brain cancers. Offered exclusively as a preclinical service by Altogen Labs, this model enables researchers to evaluate anti-cancer compounds in immunodeficient mice bearing subcutaneous human glioma tumors. It supports diverse applications in drug development, tumor biology, and translational oncology.
SF-268 cells originate from a human glioblastoma and are part of the NCI-60 cancer cell line panel. These cells are characterized by their epithelial morphology and well-documented responsiveness to a range of chemotherapeutic agents, making them ideal for comparative efficacy studies and high-throughput therapeutic screening. The SF-268 xenograft model offers a reproducible tumor take rate, controlled growth dynamics, and molecular features that support exploration of glioma-specific signaling pathways.
Role of SF-268 Cells in Glioma and Drug Sensitivity Research
SF-268 cells are often used to model intrinsic and acquired resistance to chemotherapy, particularly in the context of alkylating agents, topoisomerase inhibitors, and kinase-targeted compounds. Their relatively stable genome, in contrast to more mutated glioma lines, makes SF-268 a useful platform for identifying genotype-specific drug responses and resistance mechanisms in a consistent cellular background.
Translating these findings into in vivo experiments using SF-268 xenografts allows for validation of molecular targets, dose-response characterization, and pharmacodynamic biomarker evaluation. This model is especially useful in early-phase compound screening or in confirming mechanistic hypotheses generated from in vitro assays.
Tumor Growth Profile and Histological Features
When implanted subcutaneously into athymic nude or SCID mice, SF-268 cells give rise to tumors with predictable volume progression and moderate vascularization. The tumors display solid, non-invasive growth patterns with histological features reflective of differentiated glioma tissue. These characteristics make the model well suited for monitoring tumor inhibition over time, as well as assessing angiogenesis, apoptosis, and therapeutic impact on tumor architecture.
The relatively moderate growth kinetics of SF-268 tumors allow researchers to test extended dosing regimens or simulate clinically relevant treatment cycles. This makes the model suitable not only for acute efficacy studies but also for chronic administration studies involving low-dose combinations or sustained-release formulations.
In Vivo Applications and Experimental Flexibility
The SF-268 xenograft model offered by Altogen Labs is well suited for a wide range of preclinical research areas in oncology. It is frequently used to evaluate DNA-damaging agents and small molecules that interfere with DNA repair processes, making it a valuable system for testing radiosensitizers and chemotherapy sensitizers. This model also supports development and validation of novel drug delivery platforms such as nanoparticle-based therapeutics and liposomal formulations, which can improve drug bioavailability and tumor targeting.
In addition, the SF-268 model enables exploration of glioma-specific metabolic activity, oxidative stress responses, and cellular redox balance. Researchers often utilize this model to assess the efficacy of compounds that modulate signaling pathways known to be active in glioblastoma, including the MAPK, mTOR, and EGFR pathways. The consistent growth profile of SF-268 tumors provides an ideal framework for studies involving pathway inhibition, pharmacodynamic biomarker analysis, and survival outcome monitoring.
Altogen Labs works directly with clients to customize study protocols based on the scientific objectives of the project. Services include full tumor implantation, dosing administration, tumor volume tracking, tissue harvesting, histopathological analysis, and optional molecular profiling such as qPCR, Western blotting, or immunohistochemistry. These in vivo studies are designed to generate robust, reproducible data that can support both exploratory research and formal preclinical development programs.
Request an Instant Quote: https://altogenlabs.com/request-quote/sf-268-xenograft-model-services/
Learn more: SF-268 Xenograft Model
SF-295 Xenograft Model
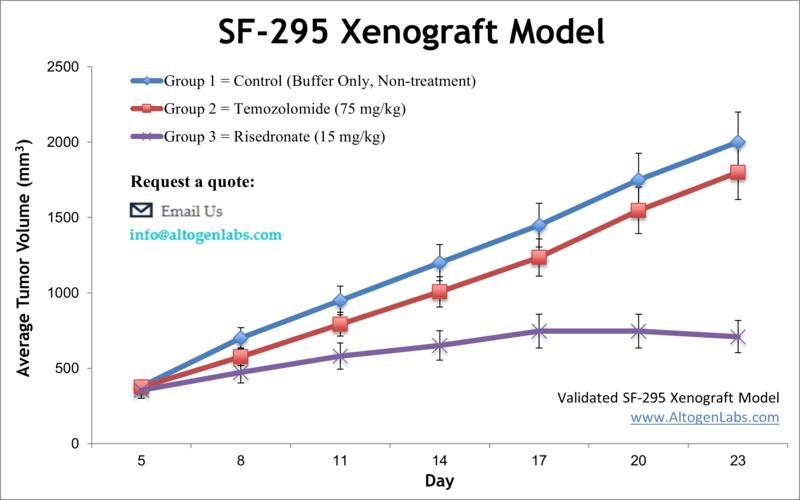
The SF-295 xenograft model is an established human glioblastoma multiforme (GBM) in vivo system used for preclinical evaluation of cancer therapeutics targeting high-grade brain tumors. Offered as a fully managed research service by Altogen Labs, this model involves subcutaneous implantation of SF-295 glioma cells into immunodeficient mice, allowing researchers to study tumor growth dynamics, drug response, and molecular changes under experimental treatments.
SF-295 cells are derived from a patient with glioblastoma and are part of the National Cancer Institute’s NCI-60 panel, which includes well-characterized cell lines for drug screening and tumor biology. These cells are known for their relatively high proliferative capacity and are frequently used to assess responses to DNA-damaging agents, kinase inhibitors, and cytotoxic compounds. When implanted in vivo, SF-295 tumors develop in a consistent, reproducible manner, making them an excellent model for evaluating therapeutic efficacy and establishing pharmacodynamic markers of tumor regression.
Scientific Context of SF-295 in Brain Cancer Research
The SF-295 cell line has been widely employed in in vitro studies of glioma biology and now plays an increasingly important role in animal-based preclinical models. These cells express a number of oncogenes and signaling regulators relevant to glioblastoma pathogenesis, including components of the PI3K/AKT and RAS/RAF/MEK pathways. Because of their documented drug sensitivity profiles, SF-295 xenografts are ideal for comparing candidate compounds against known chemotherapeutic benchmarks.
This model is frequently chosen for early-stage compound screening, toxicity studies, and dose-ranging experiments. Its predictable growth profile also makes it suitable for survival analysis and long-term monitoring of tumor suppression following single-agent or combination treatments.
Tumor Behavior and Experimental Advantages
When introduced into immunodeficient mice such as athymic nude or NOD/SCID strains, SF-295 cells generate tumors with solid, moderately aggressive growth patterns. The tumors are typically well vascularized and exhibit dense cellular architecture. Their subcutaneous localization allows for easy monitoring of tumor volume over time using caliper-based measurements, facilitating direct assessment of tumor response to treatment without the need for invasive imaging or surgical endpoints.
SF-295 xenografts generally show a reliable tumor take rate and do not produce significant necrosis or ulceration during standard study timelines. These features support the use of SF-295 in repeat-dose studies, comparative drug evaluations, and mechanistic investigations involving apoptosis, cell proliferation, or angiogenesis.
Research Applications and Custom Study Design
The SF-295 xenograft model is suitable for diverse preclinical applications including cytotoxicity profiling, pathway inhibition, anti-angiogenic studies, and exploration of chemoresistance mechanisms in glioma. It is often used to assess the therapeutic potential of novel small molecules, experimental biologics, or drug formulations designed for central nervous system tumors.
Altogen Labs provides comprehensive study execution using the SF-295 model, with services including xenograft implantation, daily animal care, dosing schedules, tumor growth tracking, tissue harvesting, and endpoint analyses. Clients can customize their studies based on intended route of drug administration, desired treatment duration, and preferred outcome measures. Available downstream analyses include histopathology, gene expression studies, protein biomarker assays, and immunohistochemistry to evaluate tumor response at the molecular level.
Request an Instant Quote: https://altogenlabs.com/request-quote/sf-295-xenograft-model-services/
Learn more: SF-295 Xenograft Model
SF-539 Xenograft Model
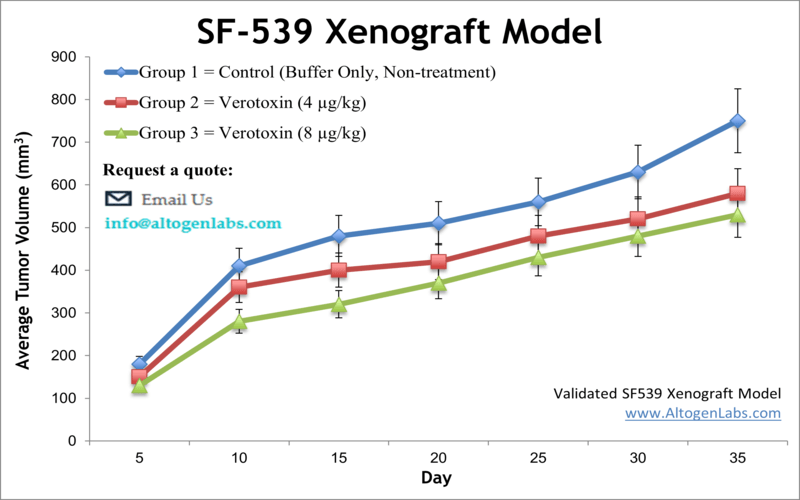
The SF-539 xenograft model is a human glioblastoma in vivo system designed for evaluating the therapeutic efficacy of experimental cancer treatments targeting aggressive brain tumors. Available as a contract research service through Altogen Labs, this model involves subcutaneous implantation of SF-539 glioma cells into immunocompromised mice, creating a reproducible and clinically relevant platform for studying tumor biology, pharmacodynamics, and anti-tumor activity.
Derived from a human GBM tumor, SF-539 cells belong to the well-known NCI-60 cell line panel used extensively in preclinical drug screening. These cells display a high proliferation rate and distinct molecular characteristics that make them especially suitable for testing cytotoxic agents, targeted therapies, and investigational compounds aimed at modulating glioma-specific signaling pathways.
Scientific Significance of SF-539 in Brain Tumor Studies
The SF-539 cell line has been used in numerous studies to explore the molecular underpinnings of glioblastoma growth, survival, and treatment resistance. In vivo, this cell line forms robust tumors with defined histological structure and reliable growth kinetics, allowing researchers to examine therapeutic effects in a controlled and quantifiable manner. The SF-539 model is well-suited for preclinical studies involving agents that disrupt DNA replication, promote apoptosis, or interfere with intracellular signaling mechanisms.
In particular, SF-539 xenografts are responsive to compounds targeting the PI3K/AKT/mTOR and MAPK pathways—signaling axes frequently deregulated in glioblastoma. The model enables detailed exploration of drug-induced tumor shrinkage, delayed progression, and downstream molecular changes. It also supports comparative studies with other glioma models for therapeutic differentiation.
Tumor Characteristics and In Vivo Performance
When engrafted into immunodeficient mice, SF-539 cells produce tumors that grow at a moderate pace and maintain consistent size progression, making the model well-suited for both short- and long-term studies. Tumors derived from SF-539 cells tend to be compact, highly cellular, and vascularized, with limited spontaneous necrosis. These properties allow researchers to monitor treatment effects over time without introducing confounding variability due to tumor instability or degeneration.
The ability to collect measurable, reproducible tumor data is particularly important for evaluating dose response, treatment durability, and drug tolerability. The SF-539 xenograft model allows for precise scheduling of compound administration and collection of tumor tissue for downstream analyses such as histology, gene expression profiling, and protein biomarker quantification.
Applications and Customizable Study Design
SF-539 xenografts are highly versatile and applicable to a wide range of preclinical oncology research goals. They can be used for testing experimental chemotherapies, pathway-specific inhibitors, immunotherapy combinations (in appropriate co-engraftment systems), and targeted delivery technologies such as nanoparticle-based platforms. The model supports investigation of cell cycle disruption, apoptosis induction, angiogenesis suppression, and resistance reversal in glioblastoma cells.
Altogen Labs offers full-service study execution using the SF-539 xenograft platform, including tumor implantation, dosing, tumor volume tracking, animal care, and customized endpoint analyses. Study designs can be tailored to match your therapeutic goals—whether you’re focused on early screening, lead optimization, or IND-enabling pharmacology studies.
Request an Instant Quote: https://altogenlabs.com/request-quote/sf-539-xenograft-model-services/
Learn more: SF-539 Xenograft Model
SK-N-AS Xenograft Model
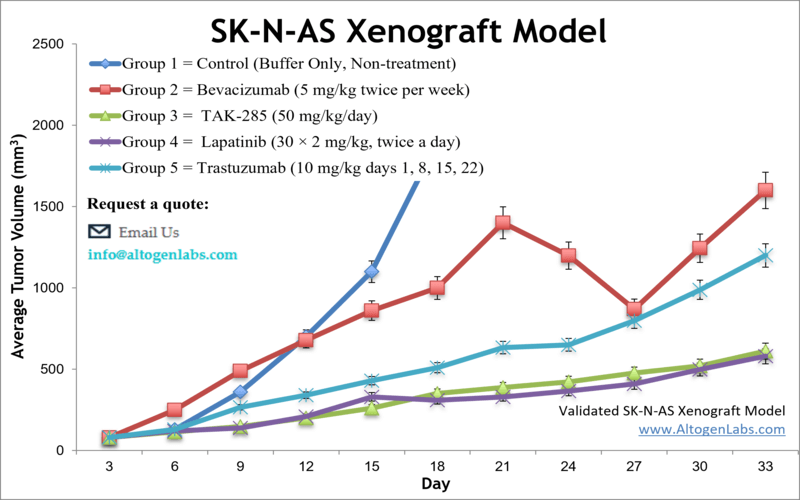
The SK-N-AS xenograft model is an in vivo system based on the human SK-N-AS neuroblastoma cell line, designed for preclinical evaluation of drug efficacy, tumor biology, and treatment response in pediatric cancer research. Provided as a full-service study platform by Altogen Labs, this model involves subcutaneous implantation of SK-N-AS cells into immunodeficient mice, forming solid, measurable tumors that mimic the characteristics of aggressive neuroblastoma in children.
SK-N-AS cells are derived from a metastatic abdominal neuroblastoma and are widely used in both in vitro and in vivo studies due to their partial differentiation, MYCN non-amplified status, and responsiveness to chemotherapy. As a xenograft model, SK-N-AS tumors allow researchers to study the effects of antitumor agents on cell proliferation, apoptosis, and metastasis-associated signaling pathways in a controlled, reproducible setting.
Relevance of SK-N-AS in Neuroblastoma Research
Neuroblastoma is one of the most common solid tumors in pediatric oncology and often presents with poor prognosis when diagnosed at later stages. The SK-N-AS cell line represents a subset of neuroblastoma cases with intermediate aggressiveness and is considered a valuable preclinical model for studying therapeutic resistance and molecular targets in MYCN–negative tumors. Unlike rapidly dividing cell lines with MYCN amplification, SK-N-AS provides a slower-growing, more treatment-sensitive tumor profile that is ideal for long-term pharmacological studies.
This model has been used to explore a range of therapeutic strategies including multi-kinase inhibition, apoptosis induction, DNA repair disruption, and immune modulation. Its consistent growth profile and well-characterized molecular features make it suitable for drug screening, mechanistic studies, and optimization of targeted therapies.
Tumor Characteristics and In Vivo Behavior
When engrafted into immunocompromised mice, SK-N-AS cells develop into compact, vascularized tumors that grow at a moderate and predictable rate. The tumors are typically well-differentiated and histologically resemble human neuroblastoma tissue, providing a physiologically relevant background for evaluating treatment efficacy. Their moderate growth dynamics make them appropriate for survival studies, repeated dosing regimens, and extended tumor monitoring timelines.
The tumors formed by SK-N-AS xenografts display low spontaneous ulceration and are easy to measure non-invasively using caliper-based methods. These features support routine monitoring of tumor volume and enable researchers to gather high-resolution data across multiple timepoints during treatment.
Applications in Preclinical Oncology Studies
The SK-N-AS xenograft model is well suited for a broad range of preclinical oncology applications, particularly those aimed at pediatric solid tumor therapeutics. It is commonly used to test cytotoxic compounds, small molecule inhibitors, RNA-based therapeutics, and novel drug delivery platforms. The model supports the study of pathways involved in neuroblastoma pathogenesis, including RAS/MAPK signaling, BCL-2-mediated apoptosis, and angiogenic regulation.
Altogen Labs offers complete in vivo study services utilizing the SK-N-AS model, including tumor cell implantation, dosing administration, animal monitoring, tumor volume tracking, and tissue harvesting for downstream analysis. Available readouts include histopathological evaluation, gene and protein expression profiling, immunohistochemistry, and pharmacodynamic biomarker detection. Study designs are fully customizable to meet the research goals of biotech, pharma, and academic clients involved in pediatric oncology and neuroblastoma drug development.
Request an Instant Quote: https://altogenlabs.com/request-quote/sk-n-as-xenograft-model-services/
Learn more: SK-N-AS Xenograft Model
SNB-19 Xenograft Model
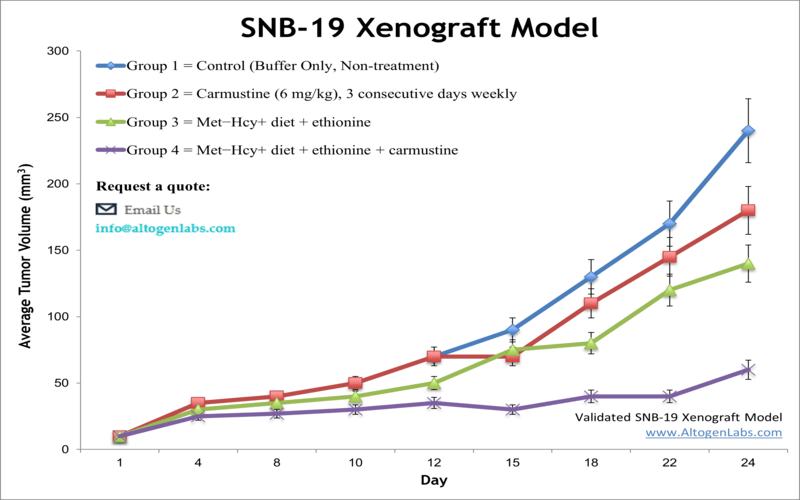
The SNB-19 xenograft model is a human glioblastoma multiforme (GBM) in vivo system used to evaluate the therapeutic potential of anticancer compounds targeting malignant brain tumors. Offered as a contract research service by Altogen Labs, this model involves the subcutaneous implantation of SNB-19 cells into immunodeficient mice, enabling the preclinical assessment of tumor inhibition, drug responsiveness, and treatment-driven molecular changes.
SNB-19 cells were derived from a human glioblastoma and are recognized for their ability to form aggressive, treatment-resistant tumors both in vitro and in vivo. These cells exhibit rapid proliferation, express astrocytic markers, and display activation of pathways commonly altered in gliomas, including PI3K/AKT, EGFR, and cell cycle regulatory genes. Their robust and reproducible tumor-forming behavior in mice makes the SNB-19 xenograft model a valuable tool for glioma research and preclinical oncology studies.
Role of SNB-19 in GBM Therapeutics Research
Glioblastoma is among the most difficult cancers to treat, characterized by its invasiveness, heterogeneity, and resistance to conventional therapies. The SNB-19 xenograft model reflects many of these clinical challenges, providing a translationally relevant platform for evaluating experimental therapies aimed at overcoming chemoresistance, targeting glioma-specific vulnerabilities, or improving drug delivery to brain-like tumor tissue.
This model is frequently selected for testing kinase inhibitors, apoptosis inducers, radiosensitizers, and drug formulations designed to penetrate the blood–brain barrier. It is also used in studies that focus on epigenetic regulation, DNA repair inhibition, and immune-modulatory mechanisms in the context of brain cancer. Because SNB-19 tumors grow aggressively in vivo, the model is ideal for studying rapid tumor progression and evaluating early treatment effects.
Tumor Characteristics and Growth Behavior
When implanted subcutaneously into athymic nude or NOD/SCID mice, SNB-19 cells generate tumors with fast and consistent growth. These tumors typically exhibit high cellular density, active vascularization, and limited necrosis under standard conditions, allowing for stable tumor volume tracking across treatment timelines. The aggressive yet predictable growth kinetics make this model appropriate for studies that require frequent measurement, defined treatment windows, or early therapeutic intervention.
Histologically, SNB-19 xenografts display features consistent with poorly differentiated gliomas, including nuclear atypia and high mitotic index. This makes the model particularly useful for mechanistic studies involving cell proliferation, apoptosis, and pathway-specific therapeutic responses.
Preclinical Applications and Study Customization
The SNB-19 xenograft model is applicable to a wide range of preclinical goals, including efficacy screening, pharmacokinetic-pharmacodynamic correlation, dose optimization, and biomarker discovery. Researchers use this model to assess antitumor activity of novel compounds, investigate combination regimens, and explore therapeutic resistance mechanisms. It is also suitable for evaluating drug delivery systems designed to enhance brain tumor targeting, such as lipid-based nanoparticles or polymer-conjugated agents.
Altogen Labs provides comprehensive xenograft study services using the SNB-19 model, including tumor establishment, drug administration, tumor volume measurement, and endpoint tissue collection. Clients can customize every aspect of their study—from administration routes and dosing frequency to downstream analysis endpoints such as histopathology, immunohistochemistry, qPCR, or western blotting. This ensures that each project is aligned with specific therapeutic hypotheses and regulatory goals.
Request an Instant Quote: https://altogenlabs.com/request-quote/snb-19-xenograft-model-services/
Learn more: SNB-19 Xenograft Model
SNB-75 Xenograft Model
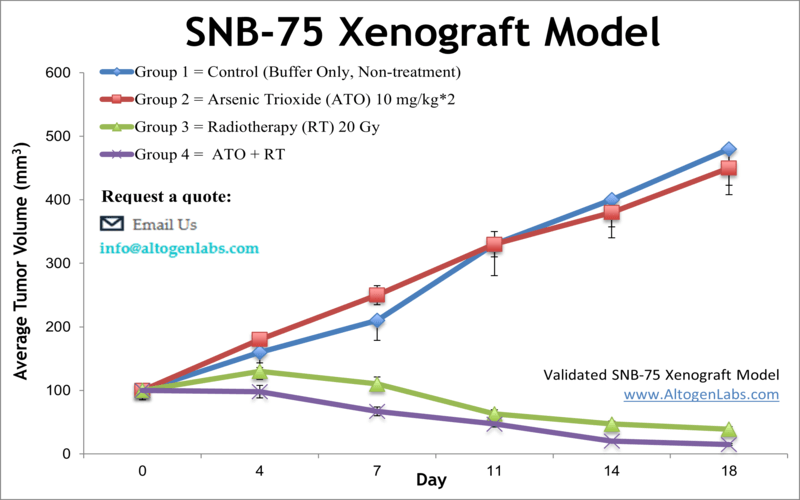
The SNB-75 xenograft model is a human glioblastoma multiforme (GBM) in vivo research system used in preclinical oncology to investigate therapeutic responses in high-grade brain tumors. Offered as a specialized service by Altogen Labs, this model involves the subcutaneous implantation of SNB-75 glioma cells into immunodeficient mice, enabling the evaluation of tumor growth, drug efficacy, and molecular outcomes in a reproducible and clinically relevant setting.
SNB-75 cells are derived from a human GBM and are part of the NCI-60 cell panel, a collection of well-characterized tumor lines widely used in anticancer drug screening. These cells are known for their epithelial morphology and moderately aggressive proliferation, making them suitable for drug studies that require both rapid and stable tumor development in vivo.
Research Relevance of SNB-75 in GBM Drug Development
The SNB-75 cell line has been used extensively to explore glioma pathophysiology, with particular focus on cell cycle regulation, DNA damage response, and apoptosis signaling. In xenograft form, it allows researchers to assess tumor response to standard-of-care and investigational therapies across multiple pathways, including EGFR inhibition, PI3K/AKT targeting, and epigenetic modulation.
This model is especially useful in bridging the gap between in vitro drug sensitivity assays and in vivo efficacy studies. It enables the analysis of therapeutic resistance, tumor suppression, and dose-dependent effects under physiological conditions that more accurately reflect clinical tumor environments. The SNB-75 xenograft system can also be applied to survival studies, pharmacodynamic evaluations, and studies involving novel drug delivery approaches to gliomas.
Tumor Characteristics and In Vivo Behavior
When implanted into athymic nude or NOD/SCID mice, SNB-75 cells form solid, vascularized tumors that exhibit a predictable growth rate. The tumors grow at a moderate pace, supporting the use of extended treatment protocols and long-term observation without the need for early endpoint termination. Histologically, SNB-75 xenografts present with high cellular density, mild necrosis, and preserved tumor architecture, facilitating downstream tissue analysis and biomarker discovery.
The reproducibility of tumor take rates and growth kinetics in this model supports the generation of high-quality data on therapeutic efficacy, drug tolerability, and biological pathway activation. This reliability makes the SNB-75 xenograft model a strong choice for studies requiring consistent tumor volume measurement, detailed response assessment, and mechanistic validation of anticancer agents.
Preclinical Applications and CRO Study Design
The SNB-75 xenograft model is well suited for a wide range of oncology research applications, including testing of small molecule inhibitors, biologics, gene therapy constructs, and combination regimens. It can be used to evaluate apoptosis inducers, angiogenesis inhibitors, and drug resistance modulators under customizable treatment schedules. Additionally, the model is compatible with delivery strategies such as liposomal formulations, micelles, and polymer-drug conjugates intended for brain tumor targeting.
Altogen Labs provides comprehensive xenograft CRO services using the SNB-75 model. Clients are supported through every step of the study, including cell implantation, treatment administration, tumor monitoring, and endpoint tissue collection. Optional downstream analyses such as histopathology, immunohistochemistry, RT-qPCR, and protein expression profiling are available to align with specific therapeutic objectives and regulatory requirements.
Request an Instant Quote: https://altogenlabs.com/request-quote/snb-75-xenograft-model-services/
Learn more: SNB-75 Xenograft Model
U87 Xenograft Model
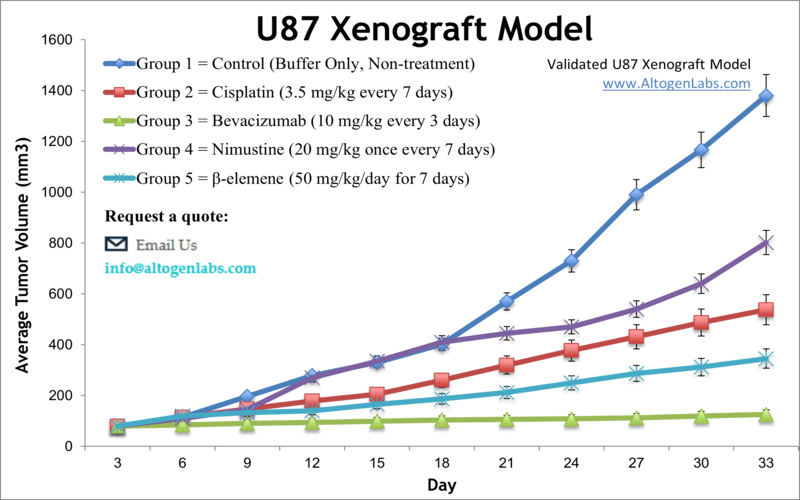
The U87 xenograft model is one of the most widely used human glioblastoma multiforme (GBM) in vivo systems for cancer research and drug development. Available as a full-service preclinical platform through Altogen Labs, this model involves subcutaneous implantation of U87 glioma cells into immunocompromised mice. The resulting tumors enable detailed evaluation of anticancer agents, including chemotherapy, targeted therapies, and novel drug delivery systems.
U87 cells, originally derived from a human malignant glioma, exhibit rapid proliferation and form well-defined, highly vascularized tumors in vivo. These features make the U87 xenograft model an established standard for early efficacy screening and mechanistic studies of glioma biology. The model provides a robust, reproducible platform for investigating tumor growth inhibition, angiogenesis suppression, and molecular signaling pathways relevant to brain cancer.
Importance of U87 in Glioblastoma Research
As one of the earliest and most extensively characterized glioma cell lines, U87 has played a key role in understanding glioblastoma pathogenesis, therapeutic resistance, and tumor microenvironment interactions. The U87 xenograft model is particularly useful for testing drugs that target cell cycle progression, growth factor receptors such as EGFR, and downstream signaling cascades including PI3K/AKT and MAPK.
Although U87 tumors grow rapidly, which can limit long-term treatment studies, this characteristic is advantageous for rapid assessment of antitumor activity and dose-response relationships. The model is also widely used to evaluate novel delivery methods such as nanoparticles and viral vectors, helping to translate in vitro findings into in vivo contexts.
Tumor Growth and Histological Features
When implanted subcutaneously into athymic nude or NOD/SCID mice, U87 cells form tumors that grow quickly and predictably. These tumors typically exhibit high cellular density, prominent vasculature, and areas of necrosis similar to aggressive human gliomas. The rapid growth rate allows for efficient tumor volume measurement using caliper methods, facilitating high-throughput screening and comparative drug studies.
Despite their aggressive nature, U87 tumors maintain consistent histopathological features across studies, making them reliable for biomarker analysis and molecular profiling. This reproducibility supports robust pharmacodynamic assessments, immunohistochemical staining, and gene expression analyses relevant to therapeutic mechanisms.
Applications and Study Customization
The U87 xenograft model supports a wide range of oncology research applications including efficacy screening of chemotherapeutics, targeted agents, radiation sensitizers, and immunotherapies. It is also well suited for evaluating combination therapies and novel formulations aimed at improving brain tumor targeting and penetration.
Altogen Labs offers comprehensive study services for U87 xenografts, including tumor implantation, dosing regimen design, tumor monitoring, and endpoint tissue collection. The lab can customize protocols to fit specific scientific goals, providing extensive downstream analyses such as histology, molecular assays, and biomarker quantification. This flexibility makes the U87 model a cornerstone in preclinical glioblastoma drug development pipelines.
Request an Instant Quote: https://altogenlabs.com/request-quote/u87-xenograft-model-services/
Learn more: U87 Xenograft Model
U118 Xenograft Model
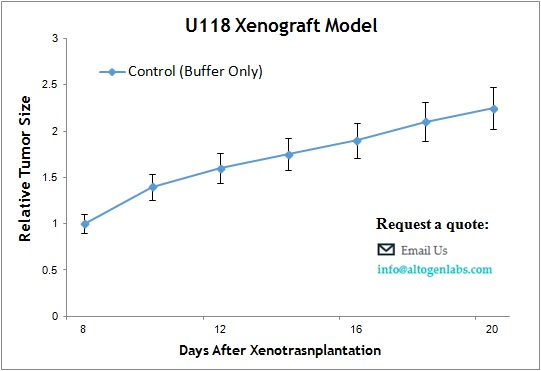
The U118 xenograft model is a human glioblastoma multiforme (GBM) in vivo system commonly used for preclinical testing of anticancer therapies targeting aggressive brain tumors. Provided as a full-service offering by Altogen Labs, this model utilizes subcutaneous implantation of U118 glioma cells into immunodeficient mice, creating a reproducible and clinically relevant tumor environment to evaluate drug efficacy, tumor growth dynamics, and molecular responses.
Derived from a malignant glioma, U118 cells exhibit characteristic features of high-grade gliomas including rapid proliferation, resistance to conventional chemotherapeutics, and aberrant signaling through oncogenic pathways such as PI3K/AKT and MAPK. The U118 xenograft model enables the assessment of investigational compounds and novel drug delivery systems under physiological conditions that mimic human glioblastoma progression.
Role of U118 in Brain Tumor Research
U118 cells are widely used in neuro-oncology research due to their consistent tumorigenic potential and well-characterized molecular profile. This model is particularly useful for studying therapeutic resistance mechanisms, cell cycle regulation, and tumor microenvironment interactions. U118 xenografts facilitate evaluation of agents targeting angiogenesis, apoptosis induction, and signal transduction pathways frequently dysregulated in glioma.
Preclinical studies using the U118 model support both early-stage compound screening and advanced pharmacodynamics research. The model’s tumor growth characteristics and molecular markers allow for detailed examination of drug effects on tumor biology, enabling translational insights that can inform clinical development strategies.
Tumor Growth and Histopathological Features
When implanted subcutaneously into athymic nude or SCID mice, U118 cells form tumors that exhibit moderate to rapid growth rates. These tumors are typically well vascularized and present with dense cellular architecture, including features such as nuclear atypia and mitotic figures consistent with glioblastoma pathology. The subcutaneous location permits non-invasive tumor volume measurements, facilitating longitudinal study designs and repeated assessments.
The reliable tumor take and growth kinetics of U118 xenografts make them suitable for dose escalation studies, survival analyses, and combination therapy experiments. Histological evaluation and biomarker analysis can be performed to investigate mechanisms of action and resistance, contributing to comprehensive preclinical datasets.
Applications and Study Customization
The U118 xenograft model is adaptable for diverse oncology research applications, including evaluation of chemotherapeutic agents, targeted therapies, immunomodulatory treatments, and experimental drug delivery platforms. It is compatible with various dosing regimens and administration routes tailored to study-specific requirements.
Altogen Labs provides full CRO services for U118 xenograft studies, encompassing tumor implantation, dosing, animal welfare monitoring, tumor measurement, and endpoint tissue collection. Clients can customize study protocols to include molecular analyses such as immunohistochemistry, gene expression profiling, and protein biomarker quantification. This flexibility ensures alignment with specific research goals and regulatory standards.
Request an Instant Quote: https://altogenlabs.com/request-quote/
Learn more: U118 Xenograft Model
U251 Xenograft Model
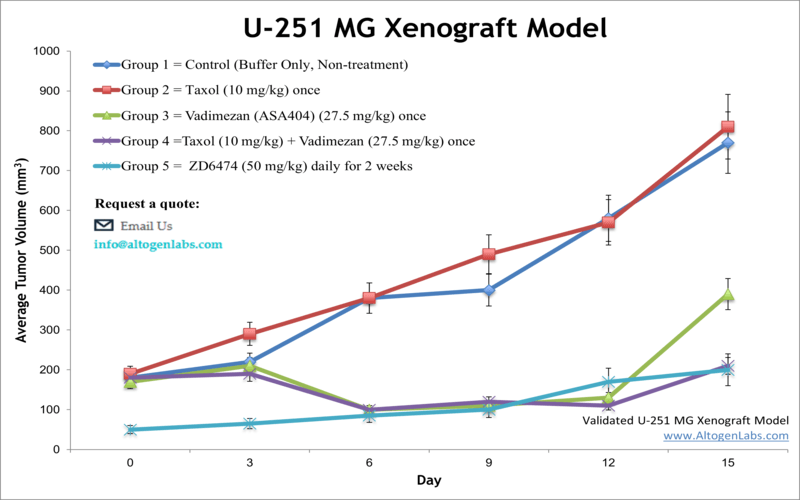
The U251 xenograft model is a widely used human glioblastoma multiforme (GBM) in vivo system that facilitates preclinical evaluation of therapeutic agents targeting aggressive brain tumors. Offered as a specialized service by Altogen Labs, this model involves subcutaneous implantation of U251 glioma cells into immunodeficient mice, providing a reliable and clinically relevant platform for studying tumor growth, drug efficacy, and molecular responses.
Originating from a human malignant glioma, U251 cells exhibit characteristic features of glioblastoma including rapid proliferation, invasive potential, and deregulated signaling through oncogenic pathways such as EGFR, PI3K/AKT, and MAPK. The U251 xenograft model enables researchers to evaluate novel chemotherapeutics, targeted agents, and drug delivery systems under physiological conditions that closely mimic human tumor biology.
Importance of U251 in Glioma Research
The U251 cell line has been extensively characterized in both in vitro and in vivo contexts, making it a cornerstone in glioblastoma research. It serves as a robust model for investigating tumor biology, treatment resistance, and therapeutic mechanisms. U251 xenografts are particularly valuable for studying the efficacy of agents that disrupt cell proliferation, induce apoptosis, or inhibit angiogenesis.
This model supports diverse research applications ranging from early-stage drug screening to advanced pharmacodynamic studies. Its tumor growth characteristics and molecular profile provide insights into therapeutic response, resistance pathways, and potential biomarkers, aiding in the translational development of glioma therapies.
Tumor Growth Profile and Histological Characteristics
When implanted subcutaneously into immunodeficient mice such as athymic nude or SCID strains, U251 cells form solid tumors that exhibit moderate to rapid growth rates. These tumors are typically well vascularized and display histological features consistent with human glioblastoma, including cellular heterogeneity, nuclear atypia, and mitotic activity.
The subcutaneous tumor model allows for straightforward monitoring of tumor volume using caliper measurements, enabling detailed assessment of tumor progression and therapeutic response over time. The reliable tumor take rate and growth kinetics facilitate dose-response studies, survival analyses, and evaluation of combination therapies.
Preclinical Applications and Study Design
The U251 xenograft model is versatile and applicable to a wide array of preclinical oncology research, including evaluation of cytotoxic drugs, molecularly targeted agents, immunotherapies, and novel delivery systems. The model is suitable for testing compounds affecting multiple signaling pathways and biological processes involved in glioma progression.
Altogen Labs offers comprehensive CRO services for U251 xenograft studies, encompassing tumor implantation, dosing administration, tumor monitoring, and endpoint tissue collection. Study protocols can be customized to meet client objectives, including detailed molecular analyses such as immunohistochemistry, gene expression profiling, and protein biomarker quantification. This flexibility ensures the generation of high-quality data to support drug development and regulatory submissions.
Request an Instant Quote: https://altogenlabs.com/request-quote/ug-251mg-xenograft-model-services/
Learn more: U251 Xenograft Model
